Aspartic Acid: Applications in Pharmaceuticals and Nutrition
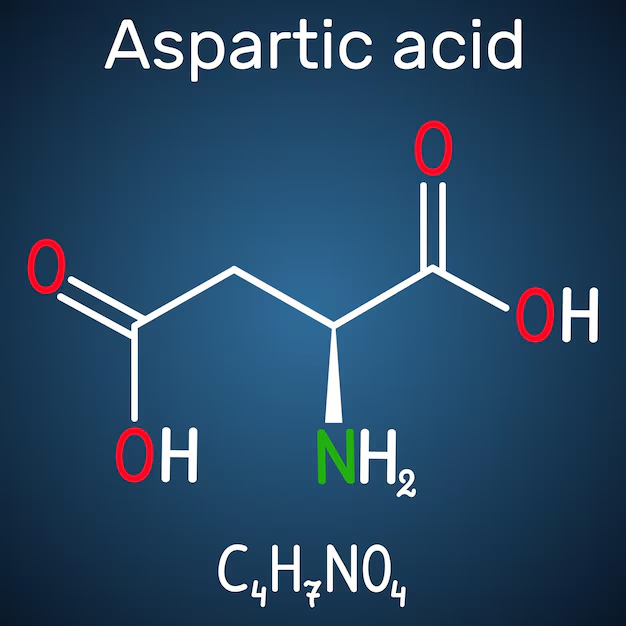
Aspartic acid (Asp or D), chemically known as 2-aminobutanedioic acid (C₄H₇NO₄), is a non-essential α-amino acid with an acidic side chain containing a carboxylic acid group. It exists in two enantiomeric forms: L-aspartic acid (the primary form incorporated into proteins) and D-aspartic acid (less common, with distinct roles in neurotransmission and hormone regulation). Aspartic acid plays vital roles in protein synthesis, energy metabolism, neurotransmission, and detoxification.
Discovered in 1827 by hydrolysis of asparagine, aspartic acid gained prominence in the 20th century for its biochemical significance and industrial applications, including as a precursor to the artificial sweetener aspartame. As of 2025, aspartic acid remains a key subject in nutrition, biochemistry, and medicine, with ongoing research exploring its therapeutic potential and safety profile. While L-aspartic acid is ubiquitous in proteins and metabolism, D-aspartic acid has garnered interest for supplements claiming hormonal benefits, though evidence is mixed.
Chemical Structure and Properties
Aspartic Acid features:
- An α-amino group (-NH₂).
- An α-carboxylic acid group (-COOH).
- A side chain -CH₂-COOH (acidic, pKa ~3.9).
At physiological pH, it is negatively charged (aspartate ion). Molecular weight: 133.10 g/mol. Solubility: Good in water, poor in organic solvents.
L- and D-forms are mirror images; L-form predominates in nature and proteins.
Sources and Biosynthesis
Natural Sources:
- Protein-rich foods: Meat, poultry, fish, eggs, dairy (high in L-form).
- Plant sources: Asparagus, avocados, sugar cane molasses, legumes.
- Dietary intake: 5-10g/day from average protein consumption.
Biosynthesis:
- L-aspartic acid synthesized via transamination of oxaloacetate (from citric acid cycle) with glutamate, catalyzed by aspartate aminotransferase (AST).
- Primary in liver, muscles, and tumor cells.
- D-aspartic acid formed by racemization of L-form or specific pathways; found in brain, testes, pituitary.
The body produces sufficient amounts, making it non-essential.
Biological Functions
- Protein Synthesis L-aspartic acid is one of 20 standard amino acids; ~5-8% of protein composition.
- Metabolism
- Urea cycle: Precursor to fumarate and arginine.
- Malate-aspartate shuttle: Transfers reducing equivalents for ATP production.
- Gluconeogenesis: From oxaloacetate.
- Neurotransmission Acts as excitatory neurotransmitter (NMDA receptor co-agonist); D-form prominent in brain development.
- Hormone Regulation D-aspartic acid stimulates LH/testosterone release in testes/pituitary.
- Detoxification Supports ammonia removal via urea cycle.
Uses and Applications
- Food Industry
- Key in aspartame synthesis (with phenylalanine methyl ester).
- Flavor enhancer, nutrient in fortified foods.
- Pharmaceuticals
- Parenteral nutrition component.
- Magnesium/potassium aspartate salts for electrolyte balance.
- Supplements
- D-aspartic acid marketed for testosterone boost, fertility (mixed evidence).
- L-aspartic acid in amino acid blends.
- Biochemistry/Medicine
- Research tool; potential in fatigue, liver support (limited human data).
Health Benefits and Research
Established:
- Protein building, energy metabolism.
- Hormone support (D-form in animal studies).
Potential:
- Fatigue reduction, athletic performance (inconclusive).
- Neuroprotection, fertility (D-aspartic acid).
Recent 2025 research: Focus on D-aspartic acid in aging, neurodegeneration; no major breakthroughs for supplements.
Safety and Side Effects
Aspartic acid from food is safe. Supplements:
- L-form: Generally safe; high doses may cause GI upset.
- D-form: 3-6g/day short-term safe; possible headaches, irritability.
- Concerns: High intake via aspartame linked to debated risks (headaches, neurological effects—largely refuted by reviews).
- Avoid excess in pregnancy, infants (animal brain defect links).
No toxicity at normal dietary levels.
Conclusion
Aspartic acid is a versatile, non-essential amino acid central to metabolism, protein synthesis, and specialized functions (particularly D-form in hormones/neurotransmission). Its industrial role in sweeteners and supplements underscores economic importance, while biological research highlights therapeutic potential. Safe in moderation from natural sources, supplemental use requires caution due to limited evidence for benefits and possible side effects at high doses. Ongoing studies continue elucidating its roles in health and disease.
More articles by ZMR Researche:
https://www.zionmarketresearch.com/de/report/water-filtration-systems-market
https://www.zionmarketresearch.com/de/report/bourbon-spirits-market
https://www.zionmarketresearch.com/de/report/logistics-picking-robots-market
https://www.zionmarketresearch.com/de/report/logistics-picking-robots-market
https://www.zionmarketresearch.com/de/report/smart-virtual-personal-assistants-market-size