Sulfonylation of Pyridine via Base-Mediated Site Selectivity
Natural goods, medicines, and agrochemicals often contain pyridine rings. For the synthesis of complex, physiologically active pyridine derivatives, direct modification of pyridine itself would be particularly desirable. Direct regioselective C-H functionalization of pyridine is difficult and typically calls for prefunctionalized starting materials with specialised protecting groups.
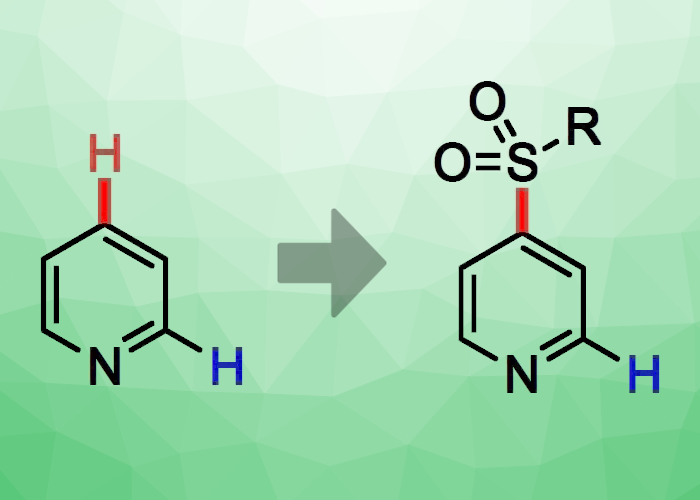
Germany’s Technical University Kaiserslautern’s Marius Friedrich and Georg Manolikakes have devised a novel base-mediated method for the direct, C4-selective sulfonylation of pyridine (pictured). This procedure begins with the standard preactivation of pyridine with triflic anhydride (TfO2), then moves on to the base-mediated addition of a nucleophlic sulfinic acid salt such sodium para-toluenesulfinate (NaTs) and finally the elimination/rearomatization step. The group’s solvent of choice was CHCl3, and their chosen base was N-methylpiperidine.
This setup allowed the team to achieve excellent regioselectivities for C4 sulfonylation. The position of the sulfonyl substituent can be guided by the chosen base. The group improved upon the technique so that sulfonylated pyridines could be built in a modular fashion. To do so, they combined phenyl lithium, activated pyridinium triflate, and the sulphur dioxide surrogate 1,4-diazabicyclo[2.2.2]octane bis(sulfonyl)oxide (DABSO) to create a C4-sulfonylated pyridine. In subsequent studies, the team plans to broaden the application of this technique to include both more N-heteroaromatics and additional nucleophile classes.